Terahertz echoes reveal the inhomogeneity of aqueous salt solutions
October 31, 2016Andrey Shalit, Saima Ahmed, Janne Savolainen and Peter Hamm use Terahertz echos to study how cations ‘structure’ water.
The change in the viscosity (ƞ) of water upon addition of simple inorganic salt is described fairly well by the semi-empirical Jones-Dole equation ƞ/ƞ˳=1+Ac1/2+Bc when ƞ˳is viscosity of water, c is a concentration and A and B are empirical coefficients. Based on the sign of the B-coefficient the ions are usually classified as “structure maker” (B>0) or “structure breakers” (B<0) based on their ability to increase/decrease viscosity upon solvation. However the consistent molecular level picture beyond this classification, namely how and to what extent ions perturbed the water structure, is still missing. The intensive experimental and computational studies applied for these systems suggesting a very diverse interpretation of the obtained data to the point that Alan Soper noticed that “the range of statements on the effect of ions perturbing the local structure of water is so wide that it can be equally concluded that either most or almost none of the water molecules in solutions are influenced by the presence of the ions…”.
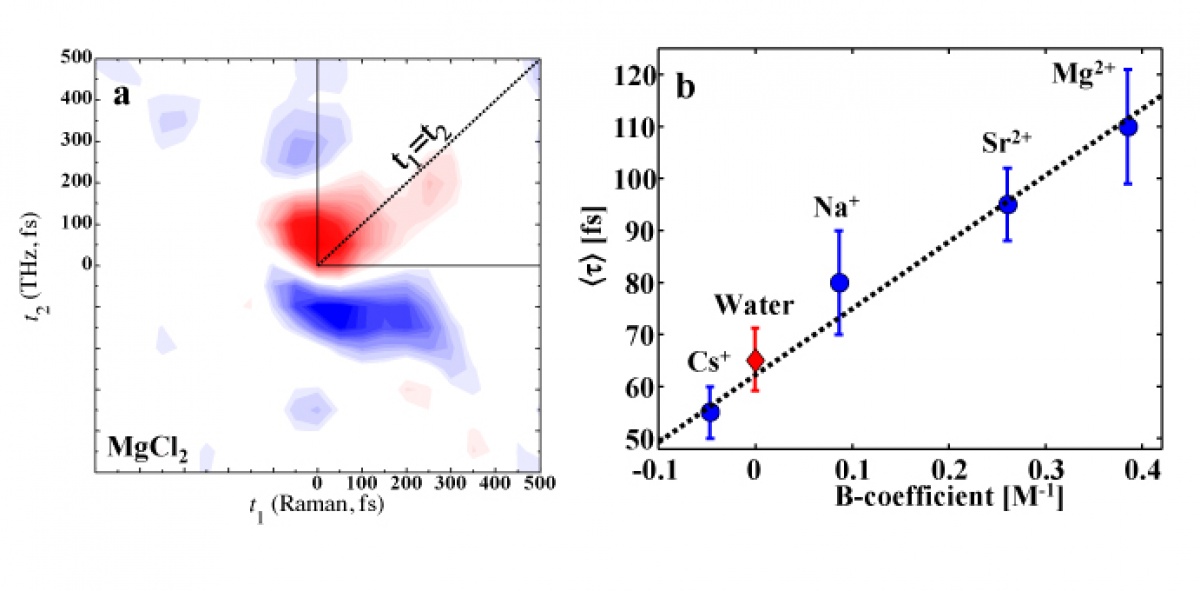
Here we apply a recently developed 2D Raman-THz spectroscopy to investigate the effect of ions on the structural and dynamical properties of water. Unlike common two-dimensional spectroscopies in IR regime, this method interrogates the hydrogen-bond stretching and bending modes of liquid water in the low frequency range, thus allowing to observation of the dynamics of the collective intermolecular motion directly. The comparison of the 2D Raman-THz response of series of chloride salts reveal the extended relaxation component along the t1=t2 diagonal in the 2D plot as the “structure making” ability of the cations are increased (Fig. 1a). As in case of conceptually similar 2D Raman spectroscopy the signal along t1=t2 reflects the degree of the inhomogeneity of the intermolecular motion. The observed trend of the increasing of the relaxation times (relative to water) qualitatively correlates with the empirical Jones-Dole B-coefficients (Fig. 1b) allowing to connect the macroscopic observable (viscosity) to microscopic hydrogen bond networks dynamics.
Given the extraordinary sensitivity of the method to detect the polarizability of the various molecular constituents, the authors believe that these experiments are the most-decisive experiments of ion solvation to date, even though currently the full information cannot be retrieved. A more-thorough interpretation of the experimental results will require massive support from theory, and we hope that our current interpretation will serve as a working hypothesis for theoretical work to come.
Reference: Shalit, A., S. Ahmed, J. Savolainen and P. Hamm (2017). Terahertz echoes reveal the inhomogeneity of aqueous salt solutions. Nature Chem. 9, 273–278 (10.1038/nchem.2642)  Shalit-2016 (2.84 MB).
Shalit-2016 (2.84 MB).

 Ursula Keller wins “Swiss Nobel” Marcel Benoist Prize
Ursula Keller wins “Swiss Nobel” Marcel Benoist Prize Farewell: the NCCR MUST ended
Farewell: the NCCR MUST ended  MUST2022 Conference
MUST2022 Conference New scientific highlights
New scientific highlights FELs of Europe prize for Jeremy Rouxel
FELs of Europe prize for Jeremy Rouxel Ruth Signorell wins Doron prize
Ruth Signorell wins Doron prize New FAST-Fellow Uwe Thumm at ETH
New FAST-Fellow Uwe Thumm at ETH International Day of Women and Girls in Science
International Day of Women and Girls in Science New scientific highlight
New scientific highlight EU XFEL Young Scientist Award for Camila Bacellar,
EU XFEL Young Scientist Award for Camila Bacellar, Prizes for Giulia Mancini and Rebeca Gomez Castillo
Prizes for Giulia Mancini and Rebeca Gomez Castillo Nobel Prize in Chemistry awarded to RESOLV Member Benjamin List
Nobel Prize in Chemistry awarded to RESOLV Member Benjamin List Hans Jakob Wörner invited to give the „New Horizons Solvay Lectures”
Hans Jakob Wörner invited to give the „New Horizons Solvay Lectures”  Unusual keynote talk at an international scientific conference
Unusual keynote talk at an international scientific conference NCCR MUST at Scientifica 2021
NCCR MUST at Scientifica 2021