Yulia Pushkar
ETH-FAST Fellow from May 15 - November 30, 2015
 | Yulia Pushkar Associate Professor of Physics Biophysics and energy research Purdue University Department of Physics and Astronomy West Lafayette, Indiana 47907-2036 USA M.S., Moscow State University, Russia 1999 Ph.D., Free University Berlin, Germany 2003 |
Tutorial topic: Bright, ultrashort X-ray pulses and what you can imagine doing with them (
 Tutorial Pushkar 23.11.2015 (19.18 MB) )
Tutorial Pushkar 23.11.2015 (19.18 MB) )Wednesday, November 25, 2015, 17.00 - 20.00, HPF G6
Abstract:
Bright, ultrashort X-ray pulses are available from synchrotron and free electron laser sources. Pulses of even shorter duration but low intensity are available via high harmonic generation. Applications of modern X-ray sources in multiple areas of science are growing and current possibilities seem to be limited only by researcher’s imagination.
In this tutorial lecture series I will cover basics of X-ray spectroscopy and X-ray imaging. X-ray spectroscopy is the main technique for analysis of electronic structures in molecules, proteins, catalysts and materials. Time resolved X-ray spectroscopy allows to uncover evolution of electronic configurations in time following the excitation. Imaging examples will include high sensitivity X-ray fluorescent imaging for studies of brain and FEL based serial crystallography. For structural techniques extended X-ray absorption fine structure (EXAFS) will be covered as a key techniques for structure analysis in solutions and disordered materials.
References
- “Serial Time-resolved crystallography of Photosystem II using a femtosecond X-ray laser” C. Kupitz et al., Nature, 2014, 513, pp 261-265.
- “Fast detection Allowing Analysis of the Electronic Structure of Metalloprotein by X-ray Emission Spectroscopy at Room Temperature” Katherine M. Davis, Brian A. Mattern, Joseph I. Pacold, Taisiya Zakharova, Dale Brewe, Irina Kosheleva, Robert W. Henning, Timothy J. Graber, Steve M. Heald, Gerald T. Seidler, Yulia Pushkar, Journal of Physical Chemistry Letters, 2012, 3, 1858–1864.
- “Identification of dopaminergic neurons of the stubstantia nigra compacta as a target of manganese accumulation” Gregory A. Robison; Brendan Sullivan; Jason R Cannon; Yulia Pushkar, Metallomics, 2015, 10.1039/C5MT00023H.
Research Interests Energy research
Sunlight provides by far the largest of all carbon-neutral energy sources. More energy from sunlight strikes the Earth in one hour (4.3 × 1020J) than all the energy consumed on the planet in a year (4.1 × 1020J). In nature, Photosynthesis accomplishes the task of light harvesting and its conversion into chemical energy. This process is extremely complex and requires interplay of many proteins and protein complexes.
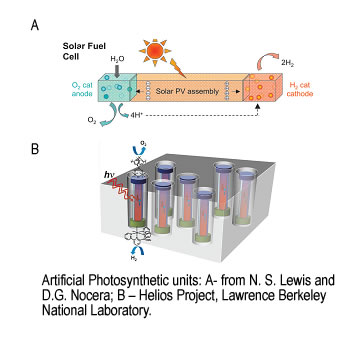
The direct conversion of visible light to chemical energy (fuel) by a synthetic device made of inorganic, organic or hybrid materials is an attractive method for harvesting sunlight in the form of fuel. Different conceptual schemes have been proposed in which sunlight is used to drive the flow of electrons and protons. Coupling the electrons and protons to catalysts breaks the bonds of water and makes the bonds of H2 and O2 to effect solar fuel production. This process requires efficient and robust water oxidation catalysts that can be incorporated into a molecular assembly or other microscopic structures or immobilized on an electrode surface.
We apply spectroscopic techniques to study biological and synthetic systems capable of converting visible light into chemically stored energy. Insight into mechanisms of their functions will help to create new, robust and efficient light-to-energy conversion units.
Metals in the brainVery recently, the importance of heavier metals, such as Fe, Cu, Mn, Zn, (0.1-0.5 mM concentrations in gray matter) for brain function was recognized. The brain utilizes these metal ions in many biochemical processes, including signalling (Cu and Zn). Abnormalities arise by two mechanisms: i) metal ion-mediated protein aggregation, ii) metal-catalyzed oxidative reactions. Proteins with redox-active metal ions have highly structured and selective active sites which prevent inappropriate electron transfer. Redox-active metals are chaperoned in the cell to prevent oxidative side reactions. It is common for neurodegenerative diseases that the binding of metal ions in the brain is highly affected. Pathological formations with increased metal concentrations were observed in brain tissues of patients with Alzheimer’s and Parkinson’s diseases.
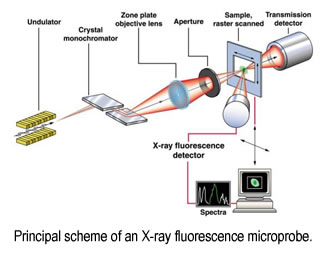
We use high resolution (micro to nano) X-ray imaging for simultaneous detection of metals (such as Fe, Cu, Mn, Zn) in neuron cell cultures and brain tissues. Subsequent collection of XAS spectra from selected areas on a sample will provide information about the redox state and ligation of the metals. There are several advantages of the proposed technique: i) it does not require staining, thus samples are unaltered prior to analysis ii) it detects all metal ions, as compared with fluorescent probes whose limited affinities result in primarily "free" ion detection; iii) it probes the redox state and the coordination environment of the metal; iv) if applied in parallel with conventional microscopy, it identifies the location of areas with increased metal concentration with respect to the tissue structure. This will result in detailed structural and functional information on metal-binding sites in normal (functional) and abnormal metalloprotein assemblies relevant for brain function. Information about the function of metals in the brain and their involvement in the development of neurodegenerative diseases will aid in the design of therapies.
Back »

 Ursula Keller wins “Swiss Nobel” Marcel Benoist Prize
Ursula Keller wins “Swiss Nobel” Marcel Benoist Prize Farewell: the NCCR MUST ended
Farewell: the NCCR MUST ended  MUST2022 Conference
MUST2022 Conference New scientific highlights
New scientific highlights FELs of Europe prize for Jeremy Rouxel
FELs of Europe prize for Jeremy Rouxel Ruth Signorell wins Doron prize
Ruth Signorell wins Doron prize New FAST-Fellow Uwe Thumm at ETH
New FAST-Fellow Uwe Thumm at ETH International Day of Women and Girls in Science
International Day of Women and Girls in Science New scientific highlight
New scientific highlight EU XFEL Young Scientist Award for Camila Bacellar,
EU XFEL Young Scientist Award for Camila Bacellar, Prizes for Giulia Mancini and Rebeca Gomez Castillo
Prizes for Giulia Mancini and Rebeca Gomez Castillo Nobel Prize in Chemistry awarded to RESOLV Member Benjamin List
Nobel Prize in Chemistry awarded to RESOLV Member Benjamin List Hans Jakob Wörner invited to give the „New Horizons Solvay Lectures”
Hans Jakob Wörner invited to give the „New Horizons Solvay Lectures”  Unusual keynote talk at an international scientific conference
Unusual keynote talk at an international scientific conference NCCR MUST at Scientifica 2021
NCCR MUST at Scientifica 2021